Announcing Lafora Therapy Town Hall
Questions about what therapies are next for the Lafora community? We are hosting a Lafora Therapy Town Hall to discuss what treatments the community can rally behind to bring from pre-clinical studies to clinical trials for our children.
Join us next Tuesday, October 28th, at 11 am PDT via Zoom.
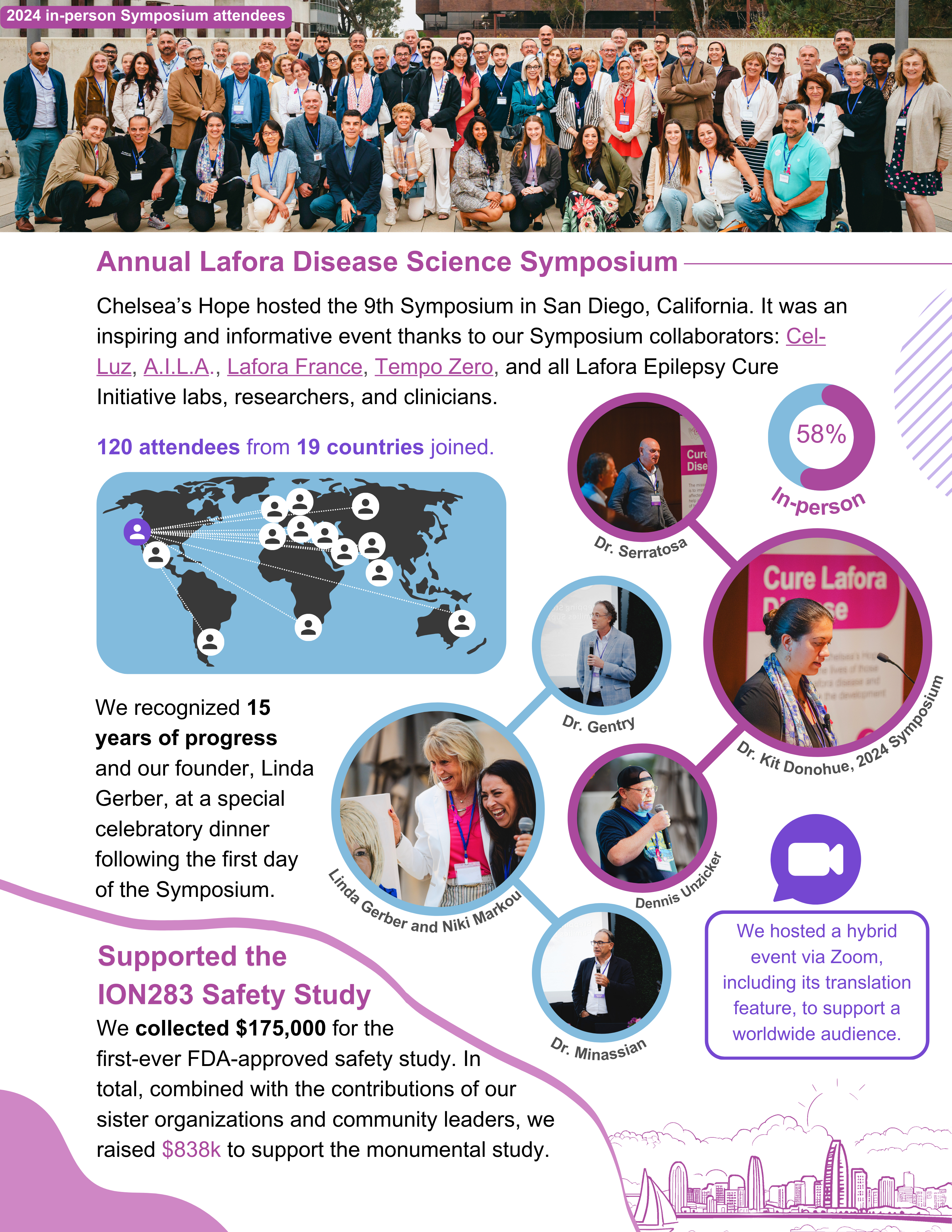
As Lafora patient organizations – Chelsea’s Hope, Cel-luz, France Lafora, A.I.L.A. – we want to be united in our message and pursuit of treatment.
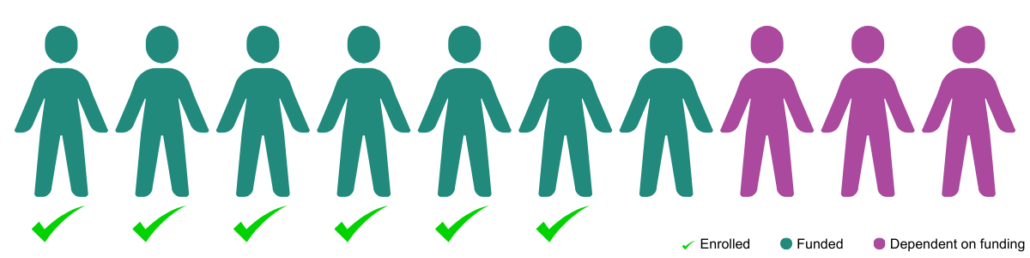
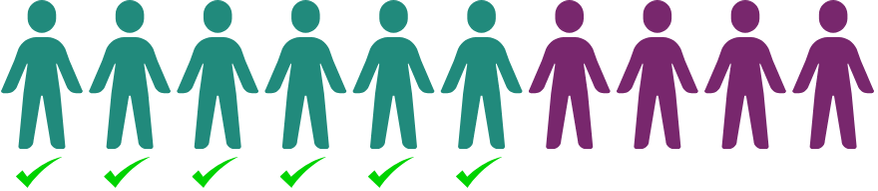
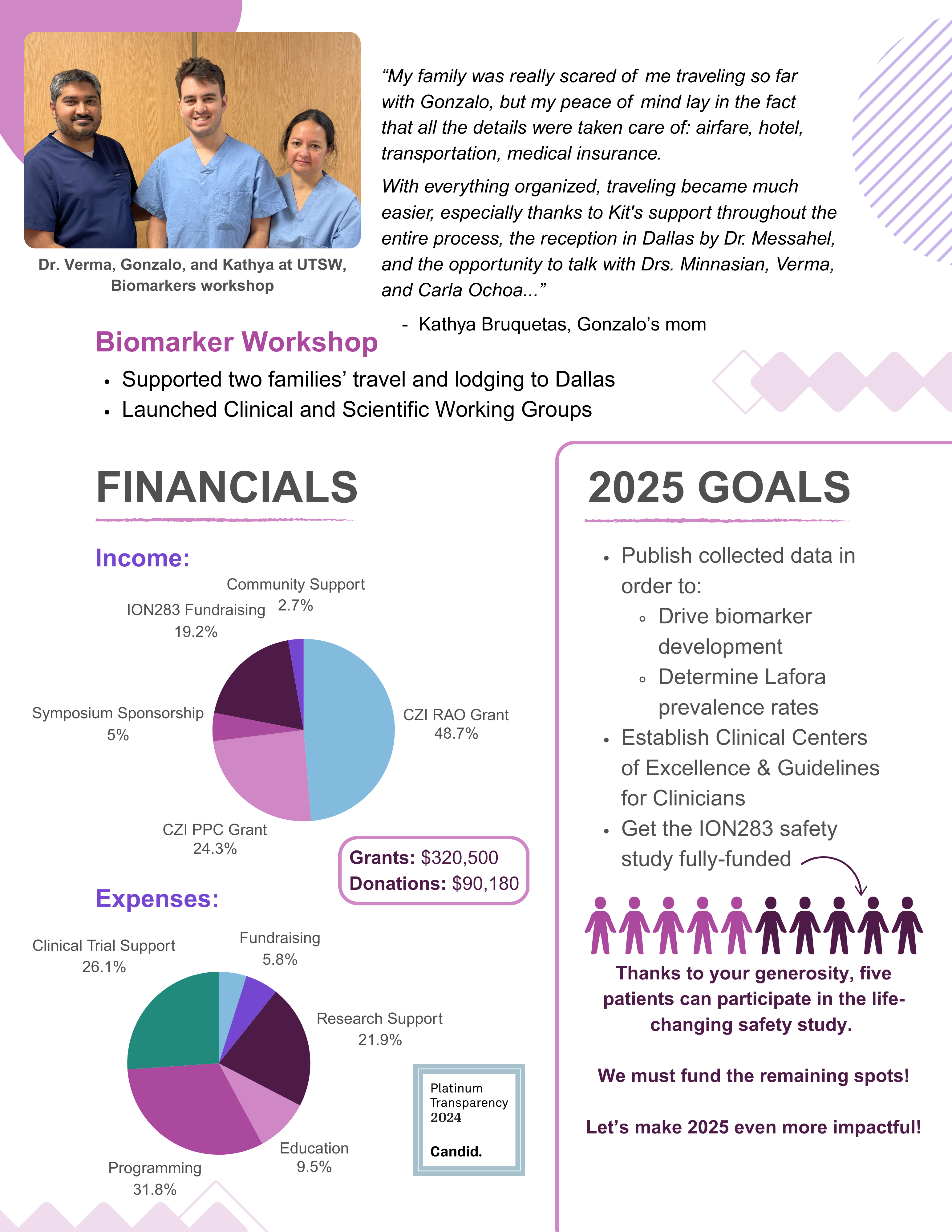
It’s been a worldwide effort to fund the safety study / phase 1 trial for ION283 at UTSW. We’ve been working towards it for years. If our patient community is going to bring another therapy forward, we must be unified in our next steps.
Families and caregivers, we hope you will be able to attend this event. Your voice matters!